How to Develop HPLC Method for Basic Compounds
Table of Contents
- Introduction and Outcome
- Basic Organic compounds
- 3 tips for separation of basic compounds
- Case study
- Procedure
- Elution Order
- Conclusion
Introduction and Outcome; HPLC Separation of Basic Compounds
HPLC separation of basic compounds; Some drug substances are basic, and to monitor the quality of these substances, an analytical method is required. In this article, I will discuss the three most important HPLC tips for the separation of basic compounds in reverse-phase chromatographic mode. After reading the article, you will be able to develop an HPLC method for basic pharmaceuticals easily. This article will also enable you to answer several questions:
- What is the principle of separation of basic Compounds?
- How to select the Mobile Phase and column for the separation of Basic Compounds?
- How to select the modifiers?
- How to optimize chromatographic conditions?
- How to optimize sample concentration and gradient?
Basic Organic Compounds
Those organic compounds tending to accept the H+ ion (proton) and remain energetically stable after the accepting of proton are called Basic Organic Compounds. For example, compounds like Aniline and Diclofenac sodium.
3 Tips for HPLC separation of basic compounds
Tip-1: Use non polar stationary phase
HPLC columns with non-polar stationary phases with high carbon loading are suitable for the separation of the basic compound in reverse-phase chromatographic mode. Hence priority should be given to C18 and C8.
Tip-2: Keep pH of the mobile basic
Try to maintain the mobile phase pH between 7.5 and 11. In this range, the tendency of basic molecules to accept protons decreases, causing these molecules to become more non-polar and separate in the reverse phase chromatographic mode.
Selection of Solvents
Methanol or Acetonitrile can be used. Considering the cost methanol should be the preferred choice
Case Study: HPLC separation of Basic molecules
Following are the structures of some basic molecules (1-(2-aminophenyl)ethan-1-one and Sodium 2-aminobenzoate):
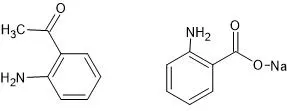
Note: The above structures have been drawn using ACD/Chemsketch (Freeware) to explain the topic
Both of the compounds can accept the proton and stabilize themselves due to the resonance effect.
Selection of HPLC column and Mobile phase
Remember to keep the pH of the mobile phase basic, preferably above 7.5. You can use a phosphate or acetate buffer at a concentration between 0.01 M to 0.03 M. For the separation, consider using a column with a C18 stationary phase, high carbon load, and more end-capping.
Methanol or Acetonitrile can be used. Considering the cost methanol should be the preferred choice.
Tip-3: Optimise the elution (gradient/isocratic) mode and Chromatographic conditions
Gradient and Isocratic mode
These compounds can be separated either in gradient or isocratic mode, but gradient mode should be the preferred choice during method development. The trial should be started with a higher aqueous phase of 75% or higher. Based on the elution of each of the molecules optimisation in the composition of the mobile phase should be done. Example:
| Time | A (0.02% K2HPO4 v/v in water pH 8.0) | B (Methanol) |
| 0 | 75 | 25 |
| 15 | 25 | 75 |
| 20 | 25 | 75 |
| 20.1 | 75 | 25 |
| 25 | 75 | 25 |
Change the composition of A and B to get a better separation. If require Replace methanol with acetonitrile.
Note:
- The above gradient table has been designed to explain this topic and it is not an actual experimental gradient.
Flow rate Optimization
Keep the flow rate between 0.5 to 1 ml/minute to get column pressure less than 2000 psi
Sample Concentration Optimisation:
Keep the sample concentration so that there should not be any column overloading and the peak should be sharp. Sample concentration can be increased or decreased based on requirement e.g sample concentration can be kept like 0.2mg/ml, 0.5mg/ml or 0.7mg/ml
Injection Volume Optimisation
Keep the Injection Volume in such a so that there should not be any column overloading and the peak
should be sharp e.g. injection volume may be 5μl, 10μl or 20μl.
Wavelength selection
Prepare the solution of each 1-(2-aminophenyl)ethan-1-one and Sodium 2-aminobenzoate and scan in a UV spectrophotometer or PDA detector. Select a wavelength where each 1-(2-aminophenyl)ethan-1-one and Sodium 2-aminobenzoate have almost equal response.
Procedure
Inject the standard solution of each 1-(2-aminophenyl)ethan-1-one and Sodium 2-aminobenzoate and generate the chromatogram. Inject the sample mixture and generate the chromatogram. Based on the elution pattern optimise the mobile phase composition and chromatographic condition to get better separation.
Calculation
Based on the requirement use area % (area normalisation method) or external standard method to give the result.
Elution Order
Since Sodium 2-aminobenzoate is relatively polar than 1-(2-aminophenyl)ethan-1-one .Therefore Sodium 2-aminobenzoate will elute first and after that 1-(2-aminophenyl)ethan-1-one elute.
Conclusion
In addition to 1-(2-aminophenyl)ethan-1-one and Sodium 2-aminobenzoate, the HPLC separation tips mentioned above can also be applied to other basic compounds. Now that you have learned about column selection, mobile phase selection, and chromatographic condition optimization for the separation of acidic compounds, I hope you can apply this knowledge during method development. That’s all for this post. Please share your thoughts and suggestions related to this post in the comments section.
Reference
- Practical HPLC – Veronika R Meyer, second edition, University of Berne, Switzerland
Abbreviations
- HPLC: High performance liquid chromatography
- C18: Octyl Decyl silane
- C8:Octyl silane
- M: Molarity
- UV: Ultraviolet
- RPC: Reverse phase chromatography



 Next Post
Next Post