Infrared (IR) or FT IR Spectrometer: How to Use
Introduction and Outcome: FTIR
Infrared (IR) or FT IR Spectrometer plays an important role in pharmaceutical analysis. Be it raw material analysis or reaction monitoring or process control or API or dosage form analysis, FTIR (Fourier Transform Interferometer) is used everywhere. This is a common tool used by all pharmaceutical companies. That’s why I decided to share my skill-based knowledge on this topic. In this article, I will discuss the principles, applications, procedures, advantages case studies and frequently asked questions of FTIR. This post will enhance your knowledge to the next level on the FTIR
WHY FTIR IS THE BACKBONE OF THE PHARMACEUTICAL ANALYSIS?
Be it raw material analysis or reaction monitoring or process control or API or dosage form analysis, FTIR (Fourier Transform Interferometer) is used everywhere. This instrument is commonly used in all pharmaceutical companies due to its fast analysis, cost-effectiveness and easy to operate. FTIR could be used for qualitative, quantitative and polymorphic analysis but it is widely used for identification test. Secondly, it is accepted by all regulatory agencies. That is why FTIR is called the backbone of pharmaceutical analysis.
IR (Infrared radiation)
The range of IR is 12800 to 10cm-1 and can be divided into:
- Near-infrared region (12800 to 4000 cm-1)
- Mid-infrared region (4000 to 400 cm-1) and
- Far-infrared region (50 to 1000 cm-1)👇
The mid-infrared region is used for IR studies.
IR spectroscopy
IR spectroscopy studies interactions between Infrared radiation and matter.
Principle of FTIR
Due to internal electronic rearrangement, atoms in the molecule do not remain fixed at their position but continuously vibrate at specific frequencies and produce IR radiation👇.
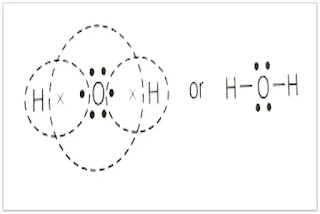
That vibrational frequency falls under the IR region. When IR radiation passes through the sample it triggers the vibration of specific molecular bonds and specific frequencies of IR radiation are absorbed. These same absorbed specific frequencies are missing from the transmitted light.
These absorbed frequencies lead to symmetric stretching, anti-symmetric stretching and bending-like vibration in the molecule👇.
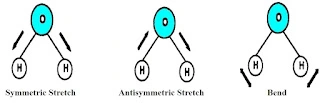
There may be other vibrational frequencies like rocking, twisting, and wagging.
Different types of bonds (e.g. double, triple) and bonds with different atoms (e.g. C-O, C-H, C-N ..) each have different vibrational frequencies. Thus transmitted light carries a lot of molecular information about the sample. The IR spectrometer uses the same transmitted to light to get the IR spectrum and in this way IR spectrum gives a lot of molecular information.
FT IR Spectrometer or Fourier transform interferometer
The centre of FTIR is the Michael interferometer and it contains:
- IR source
- Bim splitter
- Fixed mirror
- Rotating mirror &
- Detector
 |
| Michael interferometer |
FTIR Spectroscopy Instrumentation
Sample preparation:
There are several procedures for preparing the sample for FTIR analysis but the following procedures are widely used in the industries:
Solid sample
Place about 5 mg of sample and 500mg of KBr (potassium Bromide) in mortal. Grind properly using a pestle and make the pellate. Insert the pellet in a sample holder for FTIR analysis.
Liquid sample
Place about 5 mg of sample and one drop of Carbon tetrachloride.
Note:
- Other procedures can also be used for FTIR analysis
- Nowadays ATR (Attenuated Total Reflection) is used and in ATR direct sample is taken for FTIR analysis
The following procedures are adopted while performing FTIR:
- Blank IR is taken to make necessary corrections
- Standard IR is taken
- Sample IR is taken &
- The sample IR spectrum is compared with the IR spectrum👇

↓

Case study: Functional group identification
Different functional groups give IR peaks at different wavenumbers. These different functional groups are identified by FTIR. The below-mentioned molecule contains -OH(hydroxyl group), -NH-(amino group) and -CO- function groups👇

- The peak at 1644cm-1 is due to -CO-(carbonyl group
- The peaks at 1611cm-1 and 1564 cm-1 are due to the carbon-carbon double bond of the aromatic ring
- The peak at 3161cm-1 is due to -OH(hydroxyl group) and
- The peak at 3325cm-1 is due to -NH- (amino group)
Industrial applications
FTIR is widely used for Chemical Identification, Material Characterization, Surface Analysis, Qualitative Analysis and Quantitative Analysis in the following industries:
- Pharmaceutical industries
- Food industries
- Pesticide industries &
- Polymer industries
FTIR Fingerprint
It is like a chemical fingerprint. As the thumbs of two persons cannot match in the same way IR spectrum of two different molecules can not match.

FTIR is widely used in:
- Qualitative analysis like identity test
- Quantitative analysis
- Polymorphic analysis
- Structure identification and
- Quantification of crystalline water
Advantages
- Accepted by all Regulatory agencies
- Fast analysis,
- Less cost analysis and
- Specific in nature
- Non-destructive (FTIR typically doesn’t require sample preparation and does not damage the sample)
- Broad Range of Applications (It is widely used across industries like chemistry, biology, materials science, and environmental monitoring)
Conclusion:
The FTIR is very helpful in identifying and quantifying pharmaceuticals in a short period of time. Secondly, it is accepted by all regulatory agencies and that is the reason. This is all related to the use of FTIR in Pharmaceutical analysis. I hope this article has helped you understand FTIR and its applications in pharmaceutical analysis. Now you can independently perform FTIR during method development and routine analysis. You may also want to check out other articles on my blog, such as calibration of FTIR and Industrial Applications of UV Spectrophotometer
Check out this link to know answer of the following questions:
- What is the IR radiation?
- What is IR spectroscopy?
- What is the principle of FTIR?
- What are the different components of FTIR?
- What are the different Industrial applications of FTIR?
- Why FTIR is widely used in the Industries?
References
- USP<197>
Abbreviations
- IR: Infrared
- FTIR: Fourier Transform Interferometer


