Gas Chromatography and Mass Spectroscopy (GC-MS): In Drug Development
Introduction and outcome
Gas Chromatography and Mass Spectroscopy (GC-MS) is a highly sensitive, reliable, precise and accurate analytical technique and that is why it is widely used in the pharmaceuticals development for structural identification and quantification of impurities (at very low level). It is widely used for quantification of nitrosamines and other genotoxic impurities. In this article, I will share skill-based knowledge on principles, applications, advantages, disadvantages, case studies and frequently asked questions. After reading this article, you can easily develop the GC-MS method and predict the structure of unknown molecules during pharmaceuticals development.
Table of contents
- Introduction and outcome
- GC-MS Principle
- Components of GC-MS
- Two types of GC-MS Ion-source
- Difference between EI and CI mode
- Difference between EI and CI mode
- Applications of GC-MS
- Advantages
- Disadvantages
- Case studies; Identification of unknown molecule
- Conclusion
- FAQs
GC-MS Principle
GC-MS is a unique combination of two powerful techniques. The first technique is the GC instrument with a capillary column and the second technique is the mass detector. The function of GC is to separates different components from the sample mixture and send them one by one to MS detector. The function of the MS detector is to break down each component into its different fragments (minor and major). These different fragments give structural information of the component. The major fragment is also used for quantification of of the component.
Also Read: What is the difference between Analytical Method Development and Method Validation?
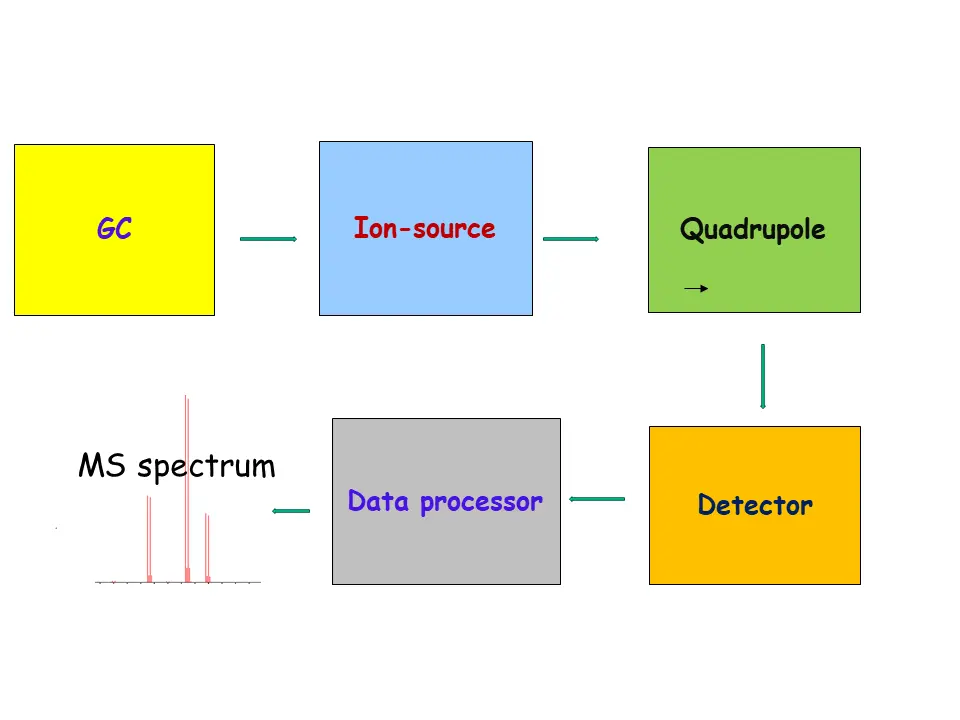
GC-MS and its Components
GC MS is the integration of GC with capillary column and MS detector
- GC with capillary column: The function of GC is to separate different components from the sample mixture and send them one by one into the MS
- MS: The function of the MS is to break down each component into its different fragments (minor and major). These different fragments give structural information of the component. The major fragment is also used for quantification of the component. The following are main components of MS:
- Interface: The function of MS-interface is to receive analyte from capillary column and send it to ion source.
- Ion-source: The function of Ion-source is to ionised the neutral molecules into into its different fragments and send it to Quadrupole. The pattern of the this fragment is highly specific and acts as a fingerprint, which is used to structure characterisation or unknown compound identification.
- Quadrupole: The quadrupole acts as mass filter and it separates ions based on M/Z ratio. It is also called mass analyser. It ensures that only fragment of specific mass to travel towards detector at a given time
- Detector: The function of the Detector is to convert each fragment into signal and send it to data processor.
- Data processor: The function of the Data processor is to convert all signals of an analyte into mass spectrum
Two types of GC-MS ion-source
The Electron ionisation (EI) and chemical ionisation (CI) are used in the most of the GC-MS instruments:
Electron ionisation (EI)
It is also called hard ionisation and it is widely used for structure elucidation. The molecule which enters into the EI-ion source gets electron bombarded of 70 EV. Due to this bombardment molecule breaks down into different fragments. The pattern of the this fragment is highly specific and acts as a fingerprint, which is used for structure characterisation of unknown compound. A stable (M+) or an unstable (M*) molecular ion may form due to this bombardment
M +e– → M+
M + e– → M* →M1+ or M2+ or M3+
Chemical ionisation (CI)
It is also called soft ionisation and it is used for mass determination. In this process, methane gas is passed at high pressure in the CI source. Since methane has very low proton affinity* and hence it can transfer proton to any molecule and that is the reason it is used in the CI source for iosisation of the molecule. CI process may go by the following mechanism:
- CH4 + e- → CH4+, CH3+, CH2+
- CH4+ + CH4 → CH5+ + CH3
- CH3+ + CH4 → C2H5+ + H2
- CH2+ + CH4 → C2H4+ + H2 and so on
Proton transfer
M +CH5+ →[M-H]+ + CH4 and so on
Note: Proton affinity for methane is 549/kJ/mol
Related topic: LCMS and Its role in pharmaceutical development
What is difference between EI and CI mode?
| EI ionisation | CI ionisation |
| It is hard ionisation | It is soft ionisation |
| It is used for structure identification of a molecule | It is used only for mass determination of a molecule |
| In EI ionisation molecule gets electron bombarded of 70 EV. | In CI ionisation, methane gas is passed at high pressure |
GC-MS spectra representation
In GC-MS, x-axis represents m/z ratio and y-axis represents relative intensity of the fragment.
What are the SIM mode and MRM mode?
SIM mode
SIM mode or selected ion monitoring mode allows mass spectrophotometer to detect specific compound with high intensity. In this mode single fragment is monitored. It is mainly used for quantification purpose.
MRM mode
MRM or multiple reaction monitoring mode is used to collect data on different fragments of the molecule. It is used for structural characterisation purpose
What are the Applications of GC-MS?
The following are the various applications of GC-MS in the Pharmaceutical industry:
- Identification of unknown components: It is very helpful in identification and characterisation of unknown impurities during drug development
- Identification of unknown organic volatile impurities: MS with GC-HS or GC-HS-MS Is very helpful in identification and characterisation of organic volatile impurities or OVI
- Mass determination of known or unknown or known molecules : It is required for structural characterisation e,g. during characterisation of standard
- Structure characterisation: MS in EI mode provides specific pattern of fragments ,which is very helpful for structural identification.
- Quantification of Genotoxic impurities at TTC level or or very low level (at ppm or ppb level) in pharmaceuticals: It is widely used in the industries for the quantification of various nitrosamine at very low level impurities
- Content test; Quantification of residual pesticides
- Drug metabolism studies: GC-MS is widely used for drug metabolism studies. By analysing biological samples such as blood and urine scientists know about the drug metabolites which is very helpful in understanding metabolic pathways
- Assay: Assay can be done by GC-MS. But it is not used for assay considering the cost of analysis.
Apart from pharmaceutical industries, GC-MS is the the following industries for various tests:
- Sports anti doping: This is the main tool used in the anti doping laboratory to test athletes urine samples for prohibited performance enhancing drugs for example antibiotic and steroids
- Research and development centre
- Cosmetic industries
- Food industries
- Fragrance industries
- Wine and beverage industries
- Polymer industries
- Pesticides industries
- Environmental monitoring
- Biological analysis
- Forensic analysis
What are the advantages of GCMS?
The following are the advantages of the GC-MS:
- High sensitivity
- Accurate, precise and reliable result
- Structure characterization of unknown compounds: Structure of unknown compounds can easily be predicted using different fragment in EI mode or using GS-MS spectrum
What are the disadvantages of GC-MS?
The following are the disadvantages of the GC-MS:
- High cost: It is a costly instrument and hence, small industries can not afford it
- Not suitable for non-volatile material compounds: It is only suitable for volatile compounds and non-volatile compounds can not be analysed
- Long analysis time: System needs longer time for stabilization. Secondly, it takes more time to to stabilize the system after change over from EI mode to CI mode and vise versa.
- Special-skill: It needs dedicated and well trained person to operate the instrument. The person must have knowledge of organic chemistry.
- Needs extra pure chemicals and gases: It needs extra pure (G-MS grade) solvents and gases to perform the analysis. It increases the cost of analysis
Case studies: Identification of unknown molecule
Use the following steps to identify the structure:
- Write down all EI fragments of the unknown molecule
- Take probable EI spectrum of unknown compound form the EI library
- Write down all solvents or chemicals used at that stage in the process
- Now correlate the fragments, probable structure obatined from EI library and solvents or chemicals used in the process
- Conclude the result and make the report
For example:
Le us consider an unknown compound gives major fragments of 60D, 45D and 15D in EI mode. In CI mode it gives mass of 60D. EI spectrum predicts closet structure of acetic acid. In the process, acetic acid is used. Therefore, unknown compound will be acetic acid.
Related topic: Impurities Control Strategies In Pharmaceuticals
Conclusion
Control, quantification, characterisation and identification of highly carcinogenic molecules is impossible without GC-MS. That is the reason GC-MS play a vital role in the pharmaceutical development. I hope this post has cleared all your doubts related to GC-MS and raised your standard to the next level.
You may also want to check out other articles on my blog, such as:
- Difference between HPLC and GC
- Need of Chromatographic Method in Drug Development
- Allowable GC Method Adjustment
- How to develop the GC method?
FAQs
What is GC-MS used for?
GC-MS is used for mass determination, identification, quantification and characterization of unknown or known compound
What is the principle of GC-MS?
GC-MS is the combination of GC with capillary column and mass. GC separate the different components of the sample mixture and mass identify each component.
What is the difference between GC and GC-MS?
GC-MS is the combination of GC with capillary column and mass. GC separate the different components of the sample mixture and mass identify each component.
Why is GC-MS best for drug analysis?
Due to its sensitivity, reliability, precision and accuracy GC-MS is best for drug analysis
What are the advantages of GC-MS?
It is highly sensitive, reliable, precise and accurate technique
What is the use of EI and CI source in GC-MS?
EI gives structural information of the molecule whereas CI gives mass of the molecule
Abbreviations
- GC: Gas chromatography
- HS: Head space
- MS: mass spectrophotometry
- EV: Electron volt
- EI: Electron ionisation
- CI: Chemical ionisation
- M/Z: Mass/charge
References
- Interpretation of mass spectra; Fred W. McLafferty
- Wikipedia



 Previous Post
Previous Post
Good eveng sir. Its interesting basic knowledge for the beginners for GC mass. I have one question in this chapter. When beam of electrons of 70eV hit the molecule it is converted into M+ or M+* ions. Now my question is that from where this one electron is released??? If we suppose and example of m-Dinitrobenzene (m/z os 168) when we performed EI GC-mass, we gets 168 now here we can assume that the formation is M+* free radical. Now where and how only one electron is released and if this is released then why not a bond is break and how we get the exact m/z value??
Thank you for your query. I an going to cover it in upcoming blog. Keep reading and writing comment. Please do mention your name.