Gas Chromatography (GC) Method Development: Easy Tips
Introduction and Background
Gas chromatography (GC) is the backbone of Pharmaceuticals development due to its reliability, sensitivity, precision fast results and cost-effectiveness. Analysis of the residual solvents and organic volatile compounds is impossible without Gas chromatography. That is why I decided to share my skill-based knowledge on this topic. In this article, I will discuss Gc principle, GC method development approaches, column selection procedure, applications, advantages, disadvantages, case studies and frequently asked questions. After reading this post, all your doubts will be cleared and you can effectively use the Gas chromatography technique in pharmaceuticals development.
Table of content
- Introduction and Background
- Principle of Gas Chromatography
- Two types of Gas Chromatography
- Two types of Gas Chromatography
- Packed columns
- Capillary columns
- Difference between Capillary column and packed column
- Components of Gas chromatography
- Type of Detectors
- Gas Chromatography Method Development and column selection
- Factors affecting GC separation
- How is the system suitability test (SST) decided in Gas chromatography?
- What are the different methods of calculation in Gas chromatography?
- Applications of Gas Chromatography
- Advantages and Disadvantages
- What are the different components of the GC method?
- Case studies
- Conclusion
- FAQs
Gas Chromatography(GC) Principle
Gas Chromatography is an analytical technique for the separation of volatile components. In Gas Chromatography, components to be separated are distributed between two phases, one of them is stationary phase and while the other is the mobile phase. In GC, mobile phase is called carrier gas. Helium and Nitrogen gases are used as carrier gas. carrier gas moves percolating through the stationary phase.
The chromatographic process occurs as a result of sorption-desorption acts during the movement of the sample components along the stationary phase and separation is caused by differences in the distribution coefficient of the sample components.
Two types of Gas Chromatography
The following are the two types of Gas chromatography:
- Gas-solid chromatography or GSC: When a solid stationary phase is used in GC, then it is called Gas-solid chromatography. Most commonly used stationary phases are molecular sieves, silica and alumina. GSC is restricted to the analysis of gases and low molecular weight hydrocarbons. Now a day GSCis not used in the industries due to its low efficiency.
- Gas-liquid chromatography or GLC: When a liquid stationary phase is used in GC, then it is called Gas-liquid chromatography.
Now in the industries, only GLC is used for its high efficiency, selectivity and reliability of result and hence in this article focus will be given on GLC.
Two types of Gas Chromatography column
The following are the two types of Gas chromatography:
- Packed columns and
- Capillary columns
Packed columns
- The packed column is a tube packed with the support material coated with a liquid stationary phase. The most widely used and readily available support material is diatomaceous earth (siliceous material).
- The column is made of glass or stainless steel. Its length is 2 to 6 meter and internal diameter is 1/4 inch to 1/8 inch.
- Efficiency of this column is low (theoretical plate 1000 to 2000).
Capillary columns
- These columns are made of fused silica and its inner wall is coated with liquid stationary phase. These columns are hollow from inside
- The length of the capillary column is 10 meter to 150 meter and its internal diameter is 0.1mm to 0.7mm.
- These columns have extremely high column efficiency (theoretical plate 10000 to 100000)
Difference between Capillary columns and packed columns
| Parameter | Capillary column | Packed column |
| Selectivity | High | Low |
| Resolution | Very good | Less |
| Column efficiency | Theoretical plate very high | Low theoretical plate |
| Tailing factor | No tailing or ideal peak | More tailing |
| Peak sharpness | Sharp peak | Broad peak |
Components of Gas chromatography
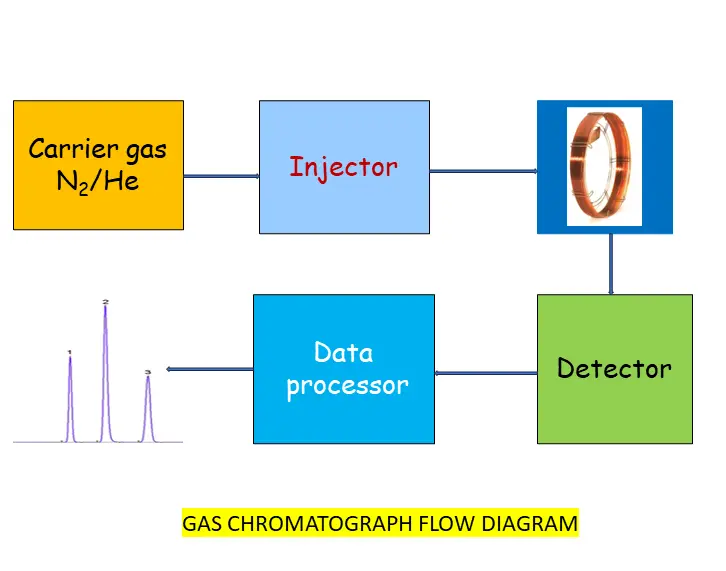
- Carrier gas: Helium and Nitrogen are used as a carrier gas. The carrier gas should be :
- Inert to avoid interaction with the sample or solvent
- Readily available
- Purity >99.9% &
- Inexpensive
- Flow control valve: Flow control valve controls the flow rate of the carrier gas
- Sample injection port: The injector injects the sample into the sample injector port. This is at high temperature and it converts the liquid sample into vapour phase. Then this vapour goes into the column. Gas tight syringes 1 microlitre to 10 microlitre are used for liquid sample and 2 ml to 10 ml syringes are used for gases.. The injector port should be hot enough to vaporise the sample rapidly but not hot so as to decompose it. The temperature of injector port is always controlled.
- GC Column: The column separates each component of the sample mixture vapour phase which is received from the injector. The each separated component goes one by one into the detector
- Column oven: The column oven maintains the column temperature as per method requirement (as per temperature program)
- Detector: The detector converts the eluted analyte into a signal and send the signal to the data processor
- Dataprocessor: Data processor converts each analyte signal (obtained from detect) into peak one by one. Hence, GC chromatogram is obtained
- Outlet
Note: If analysis is performed on flame ionisation detector in that case apart from carrier gas hydrogen and air gases are also used.
Type of Detectors
The chromatographic detector is a device which indicates and measures the amount of separated components in the carrier gas. The following types of detectors are used in the pharmaceutical industries:
- Flame ionisation detector: This detector is widely used in the pharmaceutical industries since most of the pharmaceuticals contains carbon and hydrogen atom.The molecules must have carbon and hydrogen atoms to analyse in FID detector. In this detector nitrogen or helium is used as a carrier gas. Hydrogen gas and air are used for producing the flame. Most organic of the organic compounds are readily pyrolysed when introduce into hydrogen-oxygen flame and produces ions in the process. These ions are collected at the charged electrode and resulting current passes into the data processor/integrator and peak (signal is obtained. The resulting current is the directly proportional to the concentration of the sample. This detector is highly sensitive and have a very good linearity range. Its minimum detectable limit is 1ng/ µl
- Mass detector: This is a universal detector but it is mainly used for identification of unknown impurity, structure elucidation and quantification at very low level
- Thermal conductivity detector: It is used for analysis of gases, light hydrocarbons and and those compounds that can not be analysed in FID detector.
- Electron capture detector: This detector is mainly used for analysis of volatile halo organic compounds like carbon tetrachloride and dichloromethane
- Nitrogen Phosphorus detector: This detector is used for analysis of volatile organic compounds containing Nitrogen and Phosphorus
Among above detectors, flame ionisation detector and mass detectors are widely used in the industries.
Gas Chromatography Method Development and column selection
- For separation of non polar compounds one should use non-polar stationary phase
- For the separation of polar compounds one should use polar stationary phase
Applications of Gas Chromatography
Gas Chromatography is used in the various industries such as pharmaceutical industries, food industries, petrochemicals and wine industries. The following are the various applications of GC
- Purity test: GC is widely used for purity test for volatile pharmaceuticals and other volatile chemicals
- Chiral purity test: GC with chiral column is used for the optical purity of the volatile pharmaceuticals or other volatile organic compounds
- Structural identification of unknown volatile pharmaceuticals/compounds: GC with mass (GC-MS) detector is used for structural identification of the unknown compounds
- Assay test: Gas Chromatography is used for assay test of the volatile pharmaceuticals/compounds. In GC, assay is generally performed using external standard method
- Identification test: Identification is performed by comparing the retention time of an unknown peak with known standard peak
- Content test: It is used for genotoxic impurities content in pharmaceuticals and pesticide contents in food products
- Performance enhance drugs in Atlet’s urine sample
- Environmental analysis/Air-borne pollutants analysis
- Essential oils in perfume preparation
- Forensic science: This is used for detection of explosives and analysis of arson accelerant
- Clinical analysis
Identification of different alcohols from the sample mixture using standard solution

Advantages and Disadvantages
Advantages
- The following are the advantages of the Gas Chromatography:
Fast analysis: Analysis can be performed in few minutes and that is why this technique is widely used in pharmaceuticals development - Cost-effective analysis: This is a low cost effective analysis. Even direct sample can be analysed using GC
- Reliable, precise and accurate result with high sensitivity: Gas Chromatography gives reliable, precise and accurate results with high sensitivity.
- Sharp and selective peaks: Reliable: Gas Chromatography gives sharp and selective peaks since capillary columns are used. Even a 0.2 minute difference in retention time can give base-to-base separation between the close eluting peaks
- Small sample consumption
Disadvantages
The following are the disadvantages of Gas Chromatography:
- Non-volatile pharmaceuticals/compounds can not be analysed or in other words, only volatile pharmaceuticals/compounds can be analysed by Gas Chromatography
- Compounds to be analysed should be stable in GC-operating conditions
What are the different components of the GC method?
A GC method contains the following components:
- Chemical and reagents: This section contains all the chemicals and reagents and their grade and parts numbers used in the analysis
- Instrument details: This section contains instrument and detectors details required for analysis
- GC column details: This section contains column name, its part number, make and dimension or equivalents column details. For example: DB 624, (30m x 0.53mm), 3.0 µm film thickness, make: xxxx (as applicable)
- Injector temperature:
- Detector temperature
- Oven temperature program: It contains details of the GC oven temperature
- Carrier gas: Nitrogen or Helium as required by the method
- Flow rate: It contains carrier gas required by the method
- Injection volume: It contains injection details like 1µl, 2µl or 3µl (as required by the method)
- Run time: It contains analysis run time (in minutes) required by the method
- Diluent: It contains solvent details in which sample will be prepared
- Sample, standard, sensitivity solution and system suitability preparation procedure: It contains solution preparation details with weight and glassware (e.g volumetric flask details)
- Retention time and Relative retention time table: It contains Retention time(RT) and Relative retention time (RRT), structures of different analyte components
- System suitability acceptance criteria: It contains System suitability test (SST) acceptance criteria required by the method. For example Resolution should not be less than 2, theoretical plate should not be less than 10000 etc.
- Procedure: It contains blank, standard, sample and SST injection order and calculation procedure
- Typical chromatogram: This section contains Blank, SST, sensitivity, standard and sample typical chromatogram
Case studies
- Separate Acetic acid and Ethanol form a sample mixture: Both acetic acid and ethanol are polar volatile molecules. The polarity of Ethanol acetic acid is greater than the Ethanol. Therefore these compounds can be separated using capillary columns containing polar stationary phases like Polyethylene Glycol (Carbowax)
- Separate Benzene acid and Toluene form a sample mixture: Both benzene and Toluene are the nonpolar the volatile molecules. Hence these molecules can be separated using capillary column containing nonpolar stationary phase like Dimethylpolysiloxane and 5% Diphenyl 95% dimethyl polysiloxane
Conclusion
Whether it is reaction monitoring, in-process control and release of the material, gas chromatography is needed to control the quality of the pharmaceuticals. Now I hope this post has cleared all your doubts related to GC and you can apply independently and effectively in pharmaceutical development. Any query or suggestion related to this article you can write in the comment section. You may also want to check out other articles on my blog, such as:
- Difference between HPLC and GC
- How to develop a method using GC-MS?
- Need of Chromatographic Method in Drug Development
- What is the Chiral Gas Chromatography?
- How to decide system suitability test (SST)?
- Allowable GC Method Adjustment
- How to develop the HPLC method for basic compounds?
- How to develop the HPLC method for acidic compounds?
- How to develop the HPLC method for non-polar compounds?
- What should be the Analytical method development approach?
FAQs
Read this article to know answer of the following questions
What is the meaning of GC?
What is the full form of GC?
What are the applications of gas chromatography?
What is GC and types of GC?
Which type of detectors are used in GC?
Why FID is widely used in the pharmaceutical industry?
Why is gas chromatography needed?
What is the difference between gas chromatography and liquid chromatography?
Why is hydrogen in gas chromatography?
Why is helium used as carrier gas in gas chromatography?
Abbreviations
- FID: Flame ionisation detector
- GC: Gas chromatography
- GSC: Gas solid chromatography
- GLC: Gas liquid chromatography
- MS: Mass spectrophotometer
- TCD: Thermal conductivity detector
References
- Instrumental method of analysis; willard, Merritt, Dean, Settle
- Principles of instrumental analysis; Douglas A. Skoog, F. James Holler, Stanley R, Crouch
- <621 Chromatography>



Great knowledge shared for next generation of chemist
Thank you Sir
I liked this post very much which gives brief insight about GC.
Valuable knowledge
Thank you sir for the brief details related to GC this directly helps development.
As my suggestion some points to be incorporated in the GC development PPT.
(1) Diluent selection procedure
(2) Why use a High boiler as a diluent in headspace analysis?
(3) Why use a low boiler as the diluent in GC analysis?
(4) Why use a modifier in headspace analysis (Like- NaOH, sodium chloride, anhydrous sod sulfate and sodium chloride)
(5) Oven temperature program for Isocratic and Gradient conditions during development.
(6) Injector and Detector temp limit during development
(7) What is the role of boiling point in GC analysis
Thank you. I will include all your suggestion in upcoming blog.