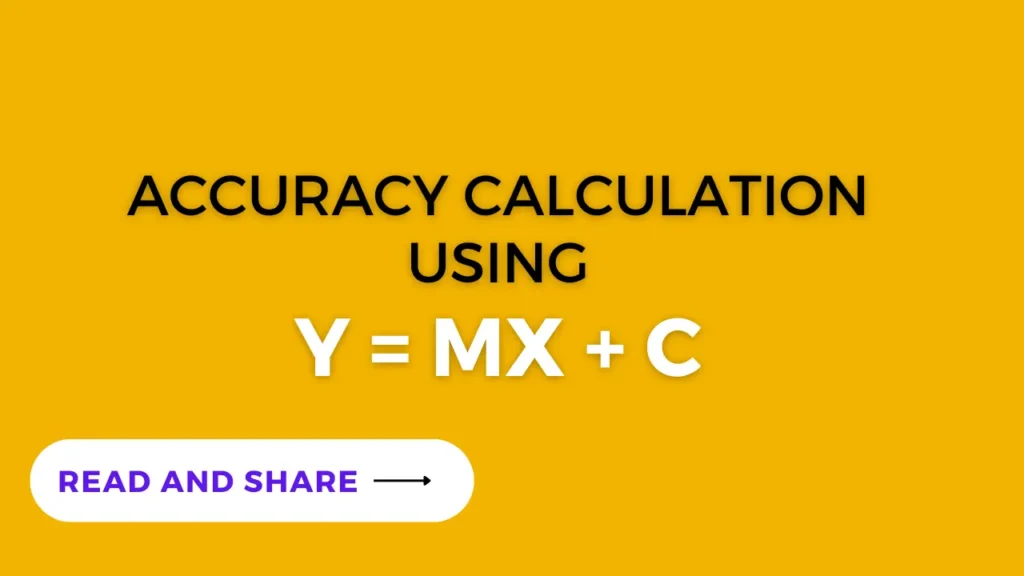
How to Perform Accuracy in Analytical Method Validation
Introduction and outcome
Accuracy is one of the important parameters of the analytical method. In this article, I will discuss procedure to perform Accuracy test with case study.
Procedure to perform accuracy

- Accuracy is determined by deviation from the linearity
- It is calculated at 50%, 100% and 150% of the specification limit
- Prepare at least five concentration between QL or 50% to 150% of the specification limit of the analyte
- Inject 50%, 100% and 150% concentrations in triplicate and inject other concentration in singlets and generate the chromatograms
- Note down the area response of each concentration
- Plot the linearity graph between concentrations (at X-axis) and their corresponding area response (at Y axis)
- Calculate slope (m), intercept (c) and correlation coefficient (R2) using MS-Excel.
- Calculate concentration for each area response using the following formula :
y= mx + c and hence x = (y-c)/m
- where: m is slope, x is the concentration, c is the intercept and y is the area response
- Calculate the accuracy at each concentration using the following formulae
- Accuracy = (Calculated concentration/True concentration) x 100
Case studies:
A drug substance D having the following specifications for related substances:
- Isomer I NMT: 1.0%
Sample concentration is 1.0mg/ml.
Standard preparation for Accuracy calculation
Since sample concentration is mg/ml or 1000 mcg/ml. The concentration of Isomer I at
- 50% will be 1000 x 0.5/100 = 5 mcg/ml
- 75% will be 1000 x 0.75/100 = 7.5 mcg/ml
- 100% will be 1000 x 1/100 = 10 mcg/ml
- 125% will be 1000 x 0.5/100 = 12.5 mcg/ml
- 150% will be 1000 x 1.5/100 = 15 mcg/ml
Use the above concentration to determine m (slope), c (intercept) and accuracy
Procedure for calculating m and c
- Inject 5 mcg/ml, 10 mcg/ml and 15 mcg/ml solutions in triplicate and remaining 7.5mcg/ml and 12.5mcg/ml solutions in singlets. Generate the chromatogram.
- Note down the area response
- Plot the linearity graph between concentrations x (at X-axis) and their corresponding area response y (at Y axis)
- Calculate slope (m), intercept (c) and correlation coefficient (R2) using MS-Excel.
| Concentration (mcg/ml) | Area response |
| 5 | 154570 |
| 7.5 | 231850 |
| 10 | 309160 |
| 12.5 | 386423 |
| 15 | 463710 |
| R2 | 1 |
| Slope (m) | 30914.12 |
| Intercept (c) | 1.4 |
Procedure for calculating x for each y using equation y= mx + c
- Calculate concentration for each y (area response) using the following formula :
- y= mx + c or
- x = (y-c)/m, where: m is slope, x is the concentration, c is the intercept and y is the area response
For y = 154570, x will be ( 231850 – 1.4)/30914.12 =5.0 mcg/ml
For y = 154600, x will be ( 154600 – 1.4)/30914.12 =5.0 mcg/ml
For y = 154200, x will be ( 154200 – 1.4)/30914.12 = 4.9 mcg/ml
and
For y = 309160, x will be ( 309160 – 1.4)/30914.12 =10 mcg/ml
For y = 463710, x will be ( 463710 – 1.4)/30914.12 =15.11 mcg/ml and similarly x can be calculated for other y (area response as well
Procedure for calculating accuracy
Calculate the accuracy at each concentration using the following formulae
Accuracy = (Calculated concentration/True concentration) x 100
| Concentration level | True concentration | Calculated concertation | Accuracy |
| 50% | 5.2 | 5.0 | 96.2% |
| 50% | 5.2 | 5.0 | 96.2 |
| 50% | 5.2 | 4.9 | 94.23 |
| Average | – | – | 95.54 |
| Average deviation | – | – | 102.04 |
Conclusion: Average deviation is less than 2.0% and hence method complies the accuracy test
Note: Similarly accuracy can also be calculated at 100% and 150% level
Conclusion
Accuracy is one of the important parameter of the Analytical method validation. Therefore, both knowledge and experience are required to perform the same. Now I hope this article has cleared all your doubts and now you can independently preform accuracy testing during method development and method validation. For any opinion or suggestions related to this article, you can write in the comment section. For any further assistance you can contact me using contact form.
You may also want to check out other articles on my blog, such as:
- Analytical method mini-validation
- Analytical technology transfer
- Ultimate Guide in Analytical Method Validation
- How to perform Solution Stability
- How to calculate Recovery in AMF?
- How to perform precision
- How to perform Linearity
- How to perform DL and QL in AMV?
- How to perform Specificity?
- How to perform Robustness?
- The best practices of Analytical Method transfer
- How to perform Forced Degradation Studies?
- How to perform Photostability?
References
- https://www.sciencedirect.com/science/article/abs/pii/S0099542805320090
- https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
Abbreviations
- mcg: microgram
- ml: milli liter
- QL: Quantitation limit
FAQs
How do you perform accuracy in Analytical method validation?
Accuracy is performed at 50%, 100% and 150% of the specification level. Standard solution is injected in triplicate at each level. Concentration is calculated using equation y = mx +c. Accuracy is calculated using formula: Accuracy = (calculated concentration/true concentration) x 100
In which case accuracy is performed?
Accuracy is performed for Active pharmaceutical ingredients or its stages. It is not applicable for dosages forms.
Disclaimer: The numerical data used in the tables or calculations are not actual data. It is designed to explain the topic.


