Method of analysis of Piperazine
Table of Contents
Assay of Piperazine
A bird’s eye view on Assay method development approach:
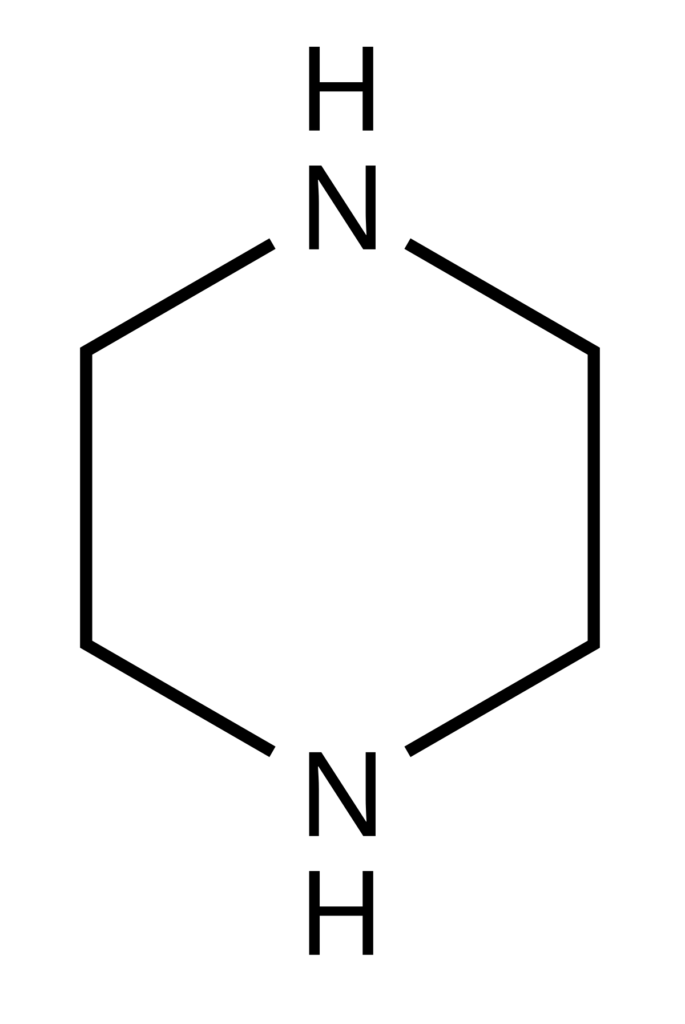
From the structure, it is clear that it has two nitrogen atoms and these nitrogen atoms make it basic in nature. Hence it can be easily quantified using acid-base titration. Its other properties are:
- Classification: Organic compound
- Molecular formula: C4H10N2
- Molecular weight: 86.136g/mol
- Uses: Treatment of worm infection
- Appearance: White crystalline powder
Reagents and chemicals required for analysis:
- 0.1N Perchloric acid in Glacial acetic acid (of known normality)
- Acetic acid
- Crystal violet indicator
Assay Procedure
Take 30 ml of acetic acid in 250 ml of conical flask. Weigh accurately about 250 mg of Pierazine sample and transfer it to the conical flask. Heat the solution with gentle shaking until the sample dissolves. Add a further 50 ml of acetic acid and cool the solution. Titrate it with 0.1N Perchloric acid using a crystal violet indicator. Perform blank titration using 80 ml of acetic acid and do necessary correction. Each of 0.1N Perchloric acid is equivalent to 0.04301gm of Piperazine.
🌟Note:
- Titrate immediately. Holding the content for a longer time may result in a faded endpoint, which may be unstable and revert back.
- Titration can also be performed using an Autotitrator without using a crystal violet indicator.
Calculation
% piperazine (OAB) = (Vx100x0.04301xNx100)/WX(100-w)
Where: V is the volume of perchloric acid consumed, N is the Normality of Perchloric acid, W is = Weight of sample in gram and w is % of water content
Impurity profile of Piperazine
A bird’s eye view on Impurity profile method development approach:
Following is the structure of Piperazine:
From the structure, it is clear that it has two nitrogen atoms and these nitrogen atoms make it basic in nature. Secondly, it does not have any chromophoric group and hence method can not be developed on HPLC using a UV detector.
Since its boiling point is 146°C and it has four oxidisable carbon atoms hence method can be developed on GC with a Flame ionisation detector using a suitable column.
Instrument
Gas chromatograph with Flame ionisation detector
Column
Dimethylpolysiloxane, 30m x 0.53mm, 5µNote: Use low bleed column such as AT-1, HP-1)
Chromatographic conditions
Initial temperature: 50°C, Initial hold time: 2 minutes, Rate:15°C/minute final Oven temperature: 150°C, Final hold time: 3 minutes, Injector temperature: 200°C, Detector temperature: 300°C, Carrier gas (He) flow: about 4 ml/minute, Injection volume: 0.2µl, Retention time: about 9 minutes
Sample concentration:
about 5.5 mg/ml in Dichloromethane
Procedure:
Inject diluent, SST and sample solution and record the chromatogram. Calculate the impurities by area normalisation method. Reject the peak due to Dichloromethane.
Abbreviations:
- m: meter
- mm: milli meter
- µm: micrometre
- µl: microlitre
- HPLC: High-performance liquid chromatography
- GC: Gas chromatography
- FID: Flame ionisation detector
- OAB: On anhydrous basis


