Understanding Thin Layer Chromatography Principle (TLC): How To Develop and Method
Introduction and Outcome: Thin Layer Chromatography (TLC)
Thin layer chromatography (TLC) is one of the most used traditional chromatographic techniques in the pharmaceutical world due to its reliability, simplicity, quick results and cost-effectiveness. Pharmaceutical development is impossible without TLC. That is why I decided to share my skill-based knowledge on this topic, In this article, you will learn the TLC principle, applications, advantages, disadvantages, method development, case studies and frequently asked questions. After reading this article all your doubts will be cleared and your knowledge will be increased to the next level
Table of content
- Introduction and Outcome
- TLC Principle
- Rf value or Retention factor value
- TLC Components
- Procedure
- Applications
- How to development the TLC Method ?
- Troubleshooting
- Advantages
- Disadvantages
- New advancement in TLC
- Conclusion
- FAQs
Thin Layer Chromatography (TLC) Principle
Thin-layer chromatography comes under complete chromatography. It contains the solid stationary phase (coated on the plate) and the liquid mobile phase. Separation is governed by the polarity principle. In other words, separation depends upon the polarity of each analyte, stationary phase and mobile phase. A spot of the sample mixture is made on the TLC plate using a syringe or capillary tube and the plate is placed in the TLC chamber containing the mobile phase. Mobile phase is moved over the TLC plate. Each Analyte of the sample mixture interacts with mobile phase as well as with the stationary phase. During interaction, the stationary phase wants to retain the analyte whereas the mobile phase wants to carry the analyte with it. The more polar compound retains more on the stationary phase (on the TLC plate) and moves slowly on the TLC plate compared to the nonpolar or less polar compound. Due to difference in the polarity, each compound interacts with with the stationary phase and the mobile phase different capacity and they separate. The relative mobility of the compound is described by the Rf or Retention factor value.
Rf value or Retention factor value
Rf=Distance of the migration of the compound /Distance moved by the mobile phase
It is the ratio of the distance covered by the compound to the distance covered by the solvent (mobile phase). Rf value is unique for each compound under specific condition such as stationary phase, temperature, thickness of adsorbent layer, amount of the spotted material and the mobile phase used
Related topic: What is the difference between TLC and HPTLC?
7 Important Components of The TLC
The following are the different components of the TLC:
- Thin Layer Chromatography Plates
- Thin Layer Chromatography (TLC) Mobile phase
- Thin Layer Chromatography (TLC) modifiers
- Thin Layer Chromatography (TLC) chamber
- Filter paper
- Thin Layer Chromatography (TLC) syringe
- Thin Layer Chromatography (TLC) UV chamber
Thin Layer Chromatography Plates
Commercial TLC plates are made of Aluminium and coated with stationary phase like Silica gel or Aluminium oxide. TLC plate can also be made using glass plate or plastic plate
Thin Layer Chromatography (TLC) Mobile phase
A solvent or mixture of solvent with different polarity is used as mobile phase. Generally, Hexane, heptane, chloroform, dichloromethane, ethyl acetate, ethanol, isopropyl alcohol are used as solvent in the mobile phase. Acetic modifiers like formic acid, acetic acid and basic modifiers like ammonia, triethyl amine and diethyl amine are also used in the mobile phases based on the polarity of the analyte.
Thin Layer Chromatography (TLC) modifiers
: Acidic modifiers like formic acid, acetic acid and basic modifiers like ammonia, triethyl amine and diethyl amine are also used in the mobile phases based on the polarity of the analyte.
Thin Layer Chromatography (TLC) chamber
The TLC chamber is made of glass and it is used to develop the TLC plate. This maintains the stable environment during analyte spot development in the TLC process. It also prevents solvent evaporation and keep entire process dust free.
Filter paper
It is used to equilibrate the TLC chamber.
Thin Layer Chromatography (TLC) syringe
It is required to apply the sample/standard spot on the TLC plate
Thin Layer Chromatography (TLC) UV chamber
- It is required to detect the analyte spot
- Colour development reagent: Reagents like Iodine vapour, Ninhydrin solution, KMnO4 solution and alkaline tetrazolium blue solutions are used for color development
How to select the TLC solvents?
TLC can be operated in Normal phase chromatographic mode (NPC) and Reverse phase chromatographic mode (RPC). But it is widely used in NPC mode. The following solvents are widely used:
- Non-Polar Solvents (Hydrophobic)
- Hexane (n-Hexane): Often used for non-polar compounds. It is commonly mixed with more polar solvents to adjust polarity.
- Petroleum Ether: A mixture of hydrocarbons, often used for non-polar to moderately polar compounds.
- Toluene: Slightly more polar than hexane and often used for moderately non-polar compounds.
- Diethyl Ether: A low-polarity solvent, often used in mixtures with hexane or other solvents.
- Polar Solvents (Hydrophilic)
- Ethyl Acetate: A relatively polar solvent used for moderately polar compounds, often in mixtures with non-polar solvents.
- Acetone: Polar, good for separating compounds with moderate polarity.
- Chloroform (CHCl₃): A moderately polar solvent, useful for separating a range of organic compounds.
- Methanol (MeOH): Very polar, often used for highly polar compounds. Methanol is also commonly mixed with other solvents like chloroform, ethyl acetate, or water.
- Water: Used in aqueous-based separations, often in combination with organic solvents.
- Mixed Solvent Systems
- Hexane / Ethyl Acetate: A common mixture, where the ratio of hexane to ethyl acetate is adjusted based on the polarity of the compounds being separated. For example, a 9:1 mixture of hexane to ethyl acetate for non-polar compounds or a 1:1 mixture for more polar compounds.
- Chloroform / Methanol: Often used for polar compounds, with varying ratios depending on the polarity of the analytes.
- Hexane / Acetone: A mixture used for compounds with intermediate polarity, often in ratios like 7:3 or 9:1.
- Other Common Solvents or Mixtures
- Dichloromethane (DCM): A moderately polar solvent, useful for a variety of organic compounds.
- Hexane / Toluene: Used for non-polar to slightly polar compounds.
- Acetonitrile (MeCN): A polar aprotic solvent, often used in gradient elution or in mixtures with other solvents.
Thin Layer Chromatography (TLC) Procedure
The following steps are involved in the TLC process;
- Make the point of spot application using pencil. Keep in mind TLC spot should be evenly spaced and should not be sink into the mobile phase.
- Apply the sample to the marked spots TLC plate using syringe or capillary
- Transfer the mobile phase in the TLC chamber
- Place the moistened (with mobile) filter paper along the inside wall of the TLC chamber. It maintain equal humidity and prevent the edge effect.
- Insert the prepared TLC plate into the chamber with sample spot facing the mobile phase.
- Close the chamber and allow sufficient time (about 40 to 60 minutes) to separate each analyte spot during TLC development
- Remove the TLC plate from the chamber and allow it to dry
- Analysed the spot using UV light or by color development (using KMnO4 or Iodine vapour)
- Calculate the Rf value
Thin Layer Chromatography (TLC) Applications
TLC is widely used for monitoring the reaction, identification test, purity test, related substances test, impurity profile test, compound purification,pesticide residue test, genotoxic impurity test and content test in the following industries:
- Research and development centre
- Pharmaceutical industries
- Pharmaceuticals formulation
- Cosmetic industries
- Pesticide industries
- Food industries
- Biochemical industries
- Polymer industries and
- Testing laboratory
How to development the TLC Method ?
In TLC, separation is governed by polarity. The mobile phased is selected based on the polarity of each analyte in the sample mixture. For polar molecules polar mobile phase is used and for non polar molecules non polar mobile phase is used. The following steps play an important role in TLC method development:
- Selection of stationary phase
- Selection of Mobile phase and
- Mobile phase optimisation
Selection of stationary phase
Silica and Alumina are commonly used as stationary phase in TLC. Therefore, the scope to play with the stationary phase during TLC development is limited.
Selection of Mobile phase
The mobile phase is designed base on the polarity of each analyte in the sample mixture . Use
- Polar mobile phase for polar molecules
- Nonpolar mobile phase for nonpolar molecules
- Modifiers in the mobile phase to control the polarity
Generally, Hexane, heptane, chloroform, dichloromethane, ethyl acetate, ethanol, isopropyl alcohol are used the mobile phase. Acidic modifiers like formic acid, acetic acid, trifluoroacetic acid and basic modifiers like ammonia, triethyl amine and diethyl amine added in the mobile phase based on the polarity of the analyte in the sample mixture.
Mobile phase optimisation
Composition of the solvent in the mobile phase is varied to get better separation between the spot.
- Increase the polar solvent (like ethanol, Isopropyl alcohol and Ethyl acetate) ratio to reduce/control the separation
- Decrease the polar solvent (like ethanol, Isopropyl alcohol and Ethyl acetate) ratio to increase the separation
Case study-1: Separation of N-(4-hydroxyphenyl)acetamide and 4-aminophenol by TLC
The following are the structure of Acetaminophen and 4 aminophenol:
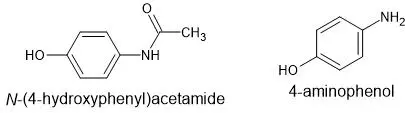
Form the above structure it is clear that both compounds are polar but 4-aminophenol is more polar compared to N-(4-hydroxyphenyl)acetamide.
- Selection of TLC stationary phase: TLC plate containing Silica gel coating can be used
- Selection of mobile phase: Both 4-aminophenol is more polar compared to N-(4-hydroxyphenyl)acetamide are containing -NH and -OH functional groups. Hence polar mobile phase with basic modifier will be suitable choice. For example ; mixture of Hexane/Heptane (non polar) and Isopropyl alcohol/Ethanol (polar) with basic modifier like Triethyl amine/ammonia solution can be used
- Mobile phase optimisation: Increase the ratio of nonpolar solvent and reduce the modfier to get better separation between the spots
- Final TLC condition: In the following TLC mobile phase condition each spot of N-(4-hydroxyphenyl)acetamide and 4-aminophenol are well separated;
- Final mobile phase: Hexane: Isopropyl alcohol:Triethylamine (90:10:0.05)
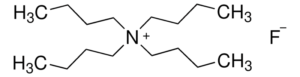
Case study 2: How to develop a TLC method for Tetrabutylammonium fluoride?
A bird’s eye view on method development approach:
- Molecular weight: C16H36FN
- Molecular weight: 261.46g/mol
Following is the structure of Tetrabutylammonium fluoride
Structure drawn in ACD/ChemSketch
From the structure, it is clear that it is a quaternary ammonium salt. It does not have any chromophore and secondly, it is an ionic compound. Therefore it will neither analyzed on HPLC nor on GC.
The only possibility is to develop a qualitative TLC method. That is why widely qualitative TLC method is used for its content in Pharmaceutical substances.
In the following TLC condition it is quantified up to 0.05% in the pharmaceuticals
TLC condition
- TLC plate: Silica Gel 60 F254
- TLC mobile phase: mixture of Dichloromethane and Methanol in the rat io of 88:12.
- TLC chamber: Vertical chamber saturated with 25% ammonium hydroxide solution
- Diluent: Dichloromethane
- Sample concentration: about 150mg/ml in Dichloromethane
- Standard: Tetrabutylammonium fluoride 0.05% ( weigh about 7.5 mg of Tetrabutylammonium fluoride and dilute to 100 ml with diluent)
- Injection volume: 5µl
- Run: about 10 cm
- Detection: iodine vapour about 25 to 30 minutes
TLC Procedure
Apply 5µl of each standard and sample solution on the TLC plate and allow the spot to dry. Place the TLC plate in the equilibrated TLC chamber. Run the mobile phase up to 10 cm on the plate. Allow the plate to dry at the room temperature in the hood. Then put the TLC plate in the iodine chamber for 25 to 30 minutes. Compare the spot of Tetrabutylammonium fluoride in the sample with standard Tetrabutylammonium fluoride .
TLC Troubleshooting
The following type of problem occurs during TLC analysis:
- Tailing in the analyte spot: If this problem occurs in the routine method then there is equilibration issue. Equilibrate the TLC system properly and then this tailing issue will be resolved
- Analyte spot is not moving from the point of application: If this type of problem occurs in the regular method then there is problem of solvent evaporation from the mobile phase. Prepare the fresh mobile phase, equilibrate the TLC system properly and then this issue will be resolved
- Analyte does not retain on the TLC plate and moves with mobile phase: If this type of problem occurs in the routine method then there is problem of mobile phase preparation. Prepare the fresh mobile phase, equilibrate the TLC system properly and then this issue will be resolved
- Distortion of the analyte’s spot: If this type of problem occurs in the routine method then there is a problem in the mobile phase and temperature of the lab. Prepare the fresh mobile phase, equilibrate the TLC system properly in temperature controlled environment and then this issue will be resolved
Advantages
The following are the TLC advantages:
- Selective technique with high separation power: Generally normal phase TLC are used in the industries and it is highly selective for qualitative analysis like identification test and purity test.
- Simple technique: It is simple technique and does not need any special skill. Anyone can perform the TLC .
- Inexpensive technique: It is cost effective analytical technique. That is reason TLC is backbone of every industry
- Quick analysis: TLC is known for its fast result. One can get result within 10 to 40 minutes. That is why it is widely used in the monitoring of the reaction.
- Versatile technique: It is versatile technique and can be used for various type of compounds such as organic and inorganic compounds. For example content of Triethylamine and tetrabutyl ammonium bromide content in pharmaceuticals is performed by TLC
- High sensitivity: It is high sensitive instrument for qualitative analysis like identification and purity test
- Sample recovery; Sample can easily be recovered after TLC analysis since volatile solvents are used in the TLC
- Visualisation: Analyte spots can easily be seen in UV chamber or by color development
- Scale up potential: TLC technique can be used for scale up after certain modification
- Regulatory acceptance: TLC technique is acceptable by all regulatory agencies in the world
Disadvantages
The following are the disadvantage of the TLC technique
- Quantitative analysis: Less suitable for quantitative analysis
- Detection limit or sensitivity: Sensitivity of the TLC is less than the other chromatographic techniques like HPLC/UPLC and GC
- Separation power: Limited stationary phases are available for the TLC and hence one can play only with the mobile phase during TLC development. That is the reason TLC has limited separation power.
New advancement in TLC
- TLC can also be performed in reverse phase chromatography mode (RPTLC). Now several new TLC plates containing C18 and C8 stationary phases are commercially available in the market.
- TLC is also used for Chiral purity using the chiral stationary phase.
RP-TLC stationary phase selection
TLC plates containing C18 and C8 stationary phases are used
RP-TLC stationary phase selection
Mixture of water and less polar organic solvents like tetrahydrofuran, methanol, ethanol, Isopropyl alcohol and acetonitrile are used.
Order of elution : Non polar analyte retin more compared to polar analyte
Advantages of RP-TLC
- Humidity does affect the result since mixture of aqueous and organic solvents are used
- Separation of analyte of different polarities such as nonpolar, less polar and extremely high polar can be possible
- RP-TLC results can be directly correlated with HPLC or UPLC
- Wide range of mobile phase selection availability and hence one can play a lot during mobile phase optimisation
Conclusion
Despite some limitations, TLC is widely used for various tests like mentoring the reaction, identifications, purity, related substances in the pharmaceutical industries due to its reliability of the result, simplicity and cost effectiveness. Now I hope this post has cleared all your doubts related to TLC and you can apply it more effectively in pharmaceutical development. For any queries or suggestion related to this article write in the comment section or contact me using contact form.
Related Topics:
- Impurities Control Strategies In Pharmaceuticals
- Analytical Method Development And Validation: How Helpful In …
- HPLC Method Development Steps For Pharmaceuticals
FAQs
What is the Thin layer chromatography?
Thin layer chromatography is the separation technique to separate the analytes from the sample mixture. It contains TLC plate coated with the stationary phase and liquid mobile phase. Separation occurs due to interaction of the analyte with the stationary phase and the mobile phase which is governed by polarity
What is the principle of Thin layer chromatography or TLC?
TLC contains the solid stationary phase (coated on the plate) and the liquid mobile phase. Separation is governed by the polarity principle.
What is the thin layer chromatography used to separate?
TLC is used to separates compound from the sample mixture. It may be westside in cosmetic sample or genotoxic impurities in the pharmaceuticals.
What are the advantages of the thin layer chromatography?’
TLC is fast, simple and cost effective technique
How thin layer chromatography is more superior than other chromatographic methods?
Due to quick result, costeffetiveness and simplicity it is more superior than other chromatography like HPLC and UPLC
How do you perform thin layer chromatography?
Prepare the sample and a make sample-spot on the TLC plate. Develop the TLC in the TLC chamber and visualize the spot in UV chamber or by colour development
Can TLC be used for quantitative analysis?
Can be used but result will not be reproducible. TLC is mainly used for qualitative analysis
How long does it take to perform a TLC analysis?
TLC is a fast analysis band can be completed within an hour
Can TLC be used for analysing volatile compounds?
Not in all cases. But some of the volatile compounds like triethylamine is analyzed by TLC
What are the thin layer chromatography plates?
Commercial TLC plates are made of Aluminium and coated with stationary phase like Silica gel or Aluminium oxide. TLC plate can also be made using glass plate or plastic plate
Abbreviations
- TLC: Thin layer chromatography
- RPTLC: Reverse phase thin layer chromatography
References
- https://www.sigmaaldrich.com/IN/en/applications/analytical-chemistry/thin-layer-chromatography
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/thin-layer-chromatography