Why is Chiral Column Chromatography important in Pharmaceuticals?
Table of Contents
- Introduction and outcome
- Why is chiral separation so important?
- Thalidomide tragedy
- Chiral and Achiral molecules
- Enantiomers, Diastereomers, Racemate and Meso-form
- Calculation for number of chiral isomers
- History behind Chirality
- Analytical-Techniques for Chiral Separation
- Chiral Stationary phase (CSP)
- Importance of Chiral Separation in Drug Development
- Applications of Chiral Separation in Pharmaceuticals
- Conclusion
- Frequently Asked Questions (FAQs)
Introduction and outcome: Chiral Column Chromatography
Chiral column chromatography plays a unique role in the separation of the chiral pharmaceuticals. Chiral pharmaceuticals have distinct spatial orientations of their atoms, which may lead to different physiological effects. This is why it is important for pharmaceutical development to take into account quality, safety and efficacy. However, the separation of chiral pharmaceuticals are challenging job for any chromatographer.
In this article, I will share my skill-based knowledge on the importance of chiral column chromatography in the pharmaceutical industry, the thalidomide effect, Chiral and Achiral molecules, the principles behind chiral chemistry, the history of chiral chemistry, techniques used for chiral separation and Chiral stationary phases. Additionally, I will provide case studies and, the answer to the frequently asked questions.
Why is Chiral Separation So Important?
More than 80% of the Active pharmaceutical ingredients are chiral. Compounds in our body are also chiral. Since chiral compounds have unique characteristics of showing handedness and hence, an isomer of the Active pharmaceuticals may be biological active or inactive or harmful. Hence control of Enantiomer/chiral isomer is necessary considering the safety and efficacy of Active pharmaceutical ingredients. That is why chiral separation is so important.
Thalidomide tragedy
The Thalidomide tragedy was one of the darkest episodes of the Pharmaceutical history. This drug was marketed in 1957 for the treatment of pregnant women developing morning sickness. This drug caused thousands of babies worldwide to be born with malformed limbs (Phocomelia). 60% of babies were died and only 40% were survived. It took about five years to find the cause of Phocomelia and it was revealed in 1962. This negative effect of Thalidomide lead to development of more structured drug regulation and control over drug use and development. Thalidomide is a chiral molecules and it contains two isomers, R-Thalidomide and S-Thalidomide. R-Thalidomide is biological active whereas S-Thalidomide is Teratogenic in nature. Initially, it was not prepared as a pure form (R-Thalidomide and it was prepared as a racemic mixture due to various limitations (like Analytical and synthetic technique limitations). The S-Thalidomide present in the Thalidomide drug was source of Thalidomide tragedy.
Understanding Chirality
Chiral and Achiral molecules
When four different groups are attached to the carbon atom then molecule becomes “asymmetric“. The carbon atom is called Chiral centre and molecule is called “Chiral molecule”.
If molecule has ≥ 2 chiral centres in that case molecule should not have any plane of symmetry to show Chirality.
“Achiral” is the reverse of the Chiral i.e. four different groups are not attached with the carbon atom.
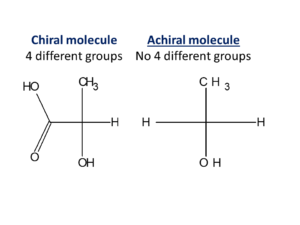
- Chiral molecules are like left right hands – they are mirror images .
- Right and left hands are non superimposable on their mirror images
- Chiral compounds are optically active; which means, they rotate plane polarised light to the left or to the right depending on their configuration.

Enantiomers, Diastereomers, Racemate and Meso-form
Enantiomers
Molecules with a chiral centre that are non-superimposable on their mirror images are called “Enantiomers”. Enantiomers have the same physical and Chemical properties. d-Lactic acid is the Enantiomer of l-Lactic acid and vice versa.
Racemate
A 1:1 mixture of enantiomers is called a Racemate/Racemic mixture.
Diastereomers
Chiral isomers that are not Enantiomers are called Diastereomers. It has different chemical and physical properties. The molecule must have ≥2 chiral centres to show this Diastereomeric properties.
Meso-form
Despite the Chiral carbon atom, the molecule is achiral. Chirality is lost by internal compensation.

Number of Chiral isomers = 2n ; where n is the number of Chiral centres
Chiral Column Chromatography
The process of separating chiral pharmaceuticals using Chiral columns/Chiral Stationary phase (CSP) is called Chiral Column Chromatography
The following Chiral column chromatography techniques are available for chiral separation:
- High-pressure liquid chromatography (HPLC)
- Gas chromatography (GC)
- Capillary electrophoresis (CE) and
- Supercritical fluid chromatography (SFC).
Among the above techniques, high-pressure liquid chromatography (HPLC) and Gas chromatography (GC) with chiral columns are widely used in industries for chiral separation
Chiral HPLC
When HPLC is used for separating chiral pharmaceuticals using chiral column then it is called chiral HPLC
Chiral GC
When GC is used for separating chiral pharmaceuticals using a chiral column then it is called chiral GC
Chiral Stationary phases (CSPs) or Chiral Columns
CSPs can be classified based on the structure of the chiral selector (CS) or the predominant interaction mechanism provided by the CSP. The following are the common types of CSPs used in the separation of chiral pharmaceuticals:
- Polysaccharide derived CSP
- Chiral crown ether
- Prickle based (Brush type)
- Antibiotic based CSP and
- Cyclodextrin based
- Protein based
Among the above phase Polysaccharide derived CSP is widely used in Pharmaceutical industries both in RPC and NPC mode
Importance of Chiral Column Chromatography in Pharmaceuticals Development
By separating enantiomers, pharmaceutical companies can develop drugs with improved efficacy and reduced side effects. Chiral separation allows for the production of pure enantiomers, ensuring that patients receive the desired therapeutic effect without the potential risks associated with the other enantiomer.
Applications of Chiral Column Chromatography in Pharmaceuticals
- Chiral separation ensures the production of enantiomerically pure drugs. This is particularly important for drugs with narrow therapeutic indexes, where even slight differences in enantiomeric composition can significantly affect efficacy and safety.
- In pharmaceutical manufacturing, chiral separation techniques are also used to purify enantiomers at scale. This ensures that the final drug product contains the desired enantiomer in the desired quantity, meeting regulatory requirements for safety and efficacy.
Case Study
A notable example highlighting the importance of chiral separation in drug safety is the case of thalidomide. In the 1960s, thalidomide was used as a sedative and anti-nausea medication for pregnant women. However, it was later discovered that one enantiomer of thalidomide caused severe birth defects, while the other enantiomer was therapeutically active. Its R-form is Biological active whereas S-form is Teratogenic in nature.
This tragic event emphasized the importance of analyzing and separating enantiomers to ensure the safety of pharmaceutical compounds. Since then, regulatory authorities have implemented strict guidelines requiring the evaluation of chiral purity for new drug candidates.
Conclusion
Chiral Column Chromatography plays a vital role in the pharmaceutical industry, ensuring the safety and efficacy of drugs. The ability to separate enantiomers allows for the development of enantiomerically pure drugs with improved therapeutic benefits and reduced side effects. Chiral Column Chromatography techniques such as HPLC, GC, CE, and SFC provide powerful tools for chiral separation, enabling the analysis, formulation, and manufacturing of chiral pharmaceutical compounds.
Now I can assume that this post must have enhanced your knowledge to the next level.
References
- https://en.wikipedia.org/wiki/Thalidomide
- https://pharmaknowledgeforum.com/high-performance-liquid-chromatography/
Abbreviations
- CSP: chiral stationary phase
- SFC: Supercritical fluid chromatography
- CE: Capillary electrophoresis
- HPLC: High performance chromatography
- RPC: Normal phase chromatography
- RPC: Reverse phase chromatography
- GC: Gas chromatography
Frequently Asked Questions (FAQs)
Q1: What is the significance of chiral purity in pharmaceutical compounds?
A1: Chiral purity refers to the presence of a single enantiomer in a pharmaceutical compound. It is crucial to ensure that drugs contain the desired enantiomer in the desired quantity to achieve the desired therapeutic effect and minimize potential side effects.
Q2: Can chiral separation improve the efficacy of existing drugs?
A2: Yes, chiral separation can potentially improve the efficacy of existing drugs by isolating the active enantiomer and eliminating the inactive or less effective enantiomer.
Q3: Are there any regulatory requirements for chiral separation in drug development?
A3: Yes, regulatory authorities, such as the FDA, require the evaluation of chiral purity for new drug candidates to ensure their safety and efficacy.
Q4: Are there any challenges associated with chiral separation?
A4: Chiral separation can be challenging due to the similarity in physical and chemical properties of enantiomers. It requires the use of specialized techniques and careful optimization to achieve efficient and selective separation.
Q5: Can chiral separation be applied to non-pharmaceutical compounds?
A5: Yes, chiral separation techniques can be applied to various industries, including agrochemicals, flavors and fragrances, and the food industry, to separate and analyze chiral compounds.



Azaming and geart learning